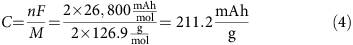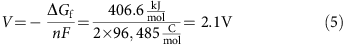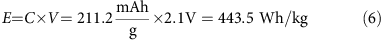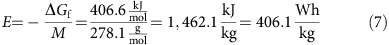Aluminum–sulfur batteries (ASBs) have attracted substantial interest due to their high theoretical specific energy density, low cost, and environmental friendliness, while the traditional sulfur cathode and ionic liquid have very fast capacity decay, limiting cycling performance because of the sluggishly electrochemical reaction and side reactions with the electrolyte. Herein, we demonstrate, for the first time, excellent rechargeable aluminum–selenium batteries (ASeBs) using a new deep eutectic solvent, thiourea-AlCl3, as an electrolyte and Se nanowires grown directly on a flexible carbon cloth substrate (Se NWs@CC) by a low-temperature selenization process as a cathode. Selenium (Se) is a chemical analogue of sulfur with higher electronic conductivity and lower ionization potential that can improve the battery kinetics on the sluggishly electrochemical reaction and the reduction of the polarization where the thiourea-AlCl3 electrolyte can stabilize the side reaction during the reversible conversion reaction of Al–Se alloying processes during the charge–discharge process, yielding a high specific capacity of 260 mAh g–1 at 50 mA g–1 and a long cycling life of 100 times with a high Coulombic efficiency of nearly 93% at 100 mA g–1. The working mechanism based on the reversible conversion reaction of the Al–Se alloying processes, confirmed by the ex situ Raman, XRD, and XPS measurements, was proposed. This work provides new insights into the development of rechargeable aluminum–chalcogenide (S, Se, and Te) batteries


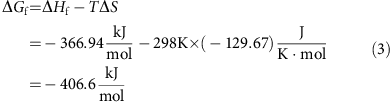
 . Therefore, the theoretical capacity of I
. Therefore, the theoretical capacity of I