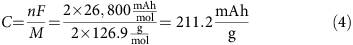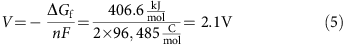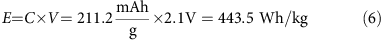經EC-Lab EIS成功擬合結果可得下表
藍色框框為deviation (dev)
紅色框框為 error ratio ( X2/ I Z I )
一般來說,dev偏差值要<1,但超過5個elements的fitting, 後面elements 的dev都可以容許比較大一點,以這範例來說R2與R3 略大於1是OK的。
而 error ratio ( X2/ I Z I ) <0.1。
一般在擬合時,會先看dev值是否<1,在查核 error ratio。
經EC-Lab EIS成功擬合結果可得下表
藍色框框為deviation (dev)
紅色框框為 error ratio ( X2/ I Z I )
一般來說,dev偏差值要<1,但超過5個elements的fitting, 後面elements 的dev都可以容許比較大一點,以這範例來說R2與R3 略大於1是OK的。
而 error ratio ( X2/ I Z I ) <0.1。
一般在擬合時,會先看dev值是否<1,在查核 error ratio。
1.基礎知識篇:
The rechargeable aluminum-ion battery (AIB) is a promising candidate for next-generation high-performance batteries, but its cathode materials require more development to improve their capacity and cycling life. We have demonstrated the growth of MoSe2 three-dimensional helical nanorod arrays on a polyimide substrate by the deposition of Mo helical nanorod arrays followed by a low-temperature plasma-assisted selenization process to form novel cathodes for AIBs. The binder-free 3D MoSe2-based AIB shows a high specific capacity of 753 mAh g–1 at a current density of 0.3 A g–1 and can maintain a high specific capacity of 138 mAh g–1 at a current density of 5 A g–1 with 10 000 cycles. Ex situ Raman, XPS, and TEM characterization results of the electrodes under different states confirm the reversible alloying conversion and intercalation hybrid mechanism during the discharge and charge cycles. All possible chemical reactions were proposed by the electrochemical curves and characterization. Further exploratory works on interdigital flexible AIBs and stretchable AIBs were demonstrated, exhibiting a steady output capacity under different bending and stretching states. This method provides a controllable strategy for selenide nanostructure-based AIBs for use in future applications of energy-storage devices in flexible and wearable electronics.
https://doi.org/10.1021/acsnano.0c02831
首先要先複製該段內文(複製的操作需建立在word有安裝endnote情況下)
然後再貼上至另一個word檔(貼上需保留功能變數)
點選Update Citations and Bibliography
如此內文中的Citations 和 尾端的Bibliography (Reference) 會自動更新。
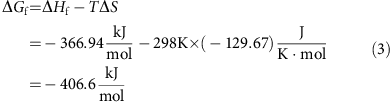
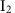 . Therefore, the theoretical capacity of I2 is
. Therefore, the theoretical capacity of I2 is